The word polymer has a Greek origin. which means many units (parts). Polymer is defined as a chemical substance of a high molecular mass formed by the combination of a large number of simple molecules, called monomers. e.g.,
Polymerisation
The process by which the monomers get combined and transformed into polymers. is known as polymerisation.
n [Monomer] → Polymer
Difference between Polymers and Macromolecules
Polymers have a molecular structure consisting chiefly or entirely of a large number of similar units bonded together. These units are called repeating units. These repeating units represent the monomers from which the polymer is made. Most of the times a macromolecule is formed due to polymerization. Then they are called polymer molecules. But some macromolecules are formed due to the chemical bonding of more atoms together. The main difference between polymer and macromolecule is that polymers contain repeating units that represent the monomers whereas not all macromolecules have a monomer in their structure.Classification of Polymers :
1.Based on Source of Origin
- Natural polymers
- Synthetic polymers
- Semisynthetic polymers
2.Based on Structure
- Linear polymers
- Branched chain polymers
- Cross-linked polymers or network polymers
3.Mode of Polymerisation
- Addition polymers
- Condensation polymers
4.Molecular Forces
- Elastomers
- Fibres
- Thermoplastics
- Thermosetting plastics
1.Based on Source of Origin
- Natural polymers[Those polymers which occur in nature. i.e., in plants or animals. are called natural polymers.e.g;silk, wool, DNA, cellulose and proteins]
- Synthetic polymers[The polymers which are prepared in the laboratory are known as synthetic polymers or man-made polymers, e.g., polythene, synthetic rubber, PVC, nylon-66, teflon, orlon etc.]
- Semisynthetic polymers [Polymers obtained by making some modification in natural polymers by artificial means, are known as semi synthetic polymers, e.g., cellulose acetate (rayon), vulcanised rubber etc.]
2.Based on Structure
- Linear polymers:[
- Branched chain polymers:[In such polymers, the monomer units are linked to form long chains with
some branched chains of different lengths with source(irregular packing). As a result of
branching, these polymers are not closely packed in space. Thus, they
have low densities, low tensile strength as well as low melting and
boiling points. Some common examples of such polymers are low density
polyethene, starch, glycogen etc.]properties:1)A low density2)Lower melting points and tensile strengths are evident, because the intermolecular bonds are weaker and require less energy to break.3)Applications:
- Cross-linked polymers or network polymers:[In such polymers, the monomer units are linked together to form three
dimensional network 3D. These are expected to be quite hard, rigid and
brittle. Examples of cross linked polymers are bakelite, glyptal,
melamine-formaldehyde polymer ,Vulcanised Rubberetc.They are mainly Thermosets Polymers] properties:1)They get degraded on Excessive Heating, though they can also resist the HIGH TEMPERATURES compared to Thermoplastics Polymers.2)High density3)Higher melting points and tensile strengths are evident, because the intermolecular bonds are strong and require high energy to break.Applications:
cross linking polymer used to boost the thermal, physical properties.Synthetic rubber used for tires is made by crosslinking rubber through the process of vulcanization This crosslinking makes them more elastic.A crossed link polymer Ethylene-vinyl-acetate is used in solar panel manufacturing.Cross-linked polymers are used in making large number of materials because they are mechanically strong and resistant to heat, wear and attack by solvents. .
3.Mode of Polymerisation
Addition polymers :A simple representation is -[A-A-A-A-A]-
The polymers formed by the polymerisation of monomers containing double or triple bonds (unsaturated compounds) are called addition polymers. Addition polymers have the same empirical formula as their monomers. Addition polymers can further be classified on the basis of the types of monomers into the following two classes:
A. Homopolymers: The polymers which are obtained by the polymerisation of a single type of monomer are called homopolymers.
Some Common Addition HomoPolymers
Name(s) Formula Monomer Properties Uses Polyethylene
low density (LDPE)–(CH2-CH2)n– ethylene
CH2=CH2soft, waxy solid film wrap, plastic bags Polyethylene
high density (HDPE)–(CH2-CH2)n– ethylene
CH2=CH2rigid, translucent solid electrical insulation
bottles, toysPolypropylene
(PP) different grades–[CH2-CH(CH3)]n– propylene
CH2=CHCH3atactic: soft, elastic solid
isotactic: hard, strong solidsimilar to LDPE
carpet, upholsteryPoly(vinyl chloride)
(PVC)–(CH2-CHCl)n– vinyl chloride
CH2=CHClstrong rigid solid pipes, siding, flooring Poly(vinylidene chloride)
(Saran A)–(CH2-CCl2)n– vinylidene chloride
CH2=CCl2dense, high-melting solid seat covers, films Polystyrene
(PS)–[CH2-CH(C6H5)]n– styrene
CH2=CHC6H5hard, rigid, clear solid
soluble in organic solventstoys, cabinets
packaging (foamed)Polyacrylonitrile
(PAN, Orlon, Acrilan)–(CH2-CHCN)n– acrylonitrile
CH2=CHCNhigh-melting solid
soluble in organic solventsrugs, blankets
clothingPolytetrafluoroethylene
(PTFE, Teflon)–(CF2-CF2)n– tetrafluoroethylene
CF2=CF2resistant, smooth solid non-stick surfaces
electrical insulationPoly(methyl methacrylate)
(PMMA, Lucite, Plexiglas)–[CH2-C(CH3)CO2CH3]n– methyl methacrylate
CH2=C(CH3)CO2CH3hard, transparent solid lighting covers, signs
skylightsPoly(vinyl acetate)
(PVAc)–(CH2-CHOCOCH3)n– vinyl acetate
CH2=CHOCOCH3soft, sticky solid latex paints, adhesives cis-Polyisoprene
natural rubber–[CH2-CH=C(CH3)-CH2]n– isoprene
CH2=CH-C(CH3)=CH2soft, sticky solid requires vulcanization
for practical usePolychloroprene (cis + trans)
(Neoprene)–[CH2-CH=CCl-CH2]n– chloroprene
CH2=CH-CCl=CH2tough, rubbery solid synthetic rubber
oil resistant
William ReuschMichigan State University]
B.Copolymers:The polymers which are obtained by the polymerisation of two or more monomers are called copolymers.
| Monomer A | Monomer B | Copolymer | Uses |
|---|---|---|---|
| H2C=CHCl | H2C=CCl2 | Saran | films & fibers |
| H2C=CHC6H5 | H2C=C-CH=CH2 | SBR styrene butadiene rubber | tires |
| H2C=CHCN | H2C=C-CH=CH2 | Nitrile Rubber | adhesives hoses |
| H2C=C(CH3)2 | H2C=C-CH=CH2 | Butyl Rubber | inner tubes |
| F2C=CF(CF3) | H2C=CHF | Viton | gaskets |
Polymerization Methods
Addition polymerization
is the successive addition of alkene monomers to one another. The
addition reaction may occur by way of
- radical, (Link)
- cationic, (Link)
- or anionic intermediates.(Link)
Condensation polymers: The polymers which are formed by the combination of monomers with the elimination of small molecules such as water, alcohol, hydrogen chloride etc., are known as condensation polymers, e.g., polyester Dacron and nylon 6,6 is formed by the condensation of hexamethylene diamine with adipic acid.
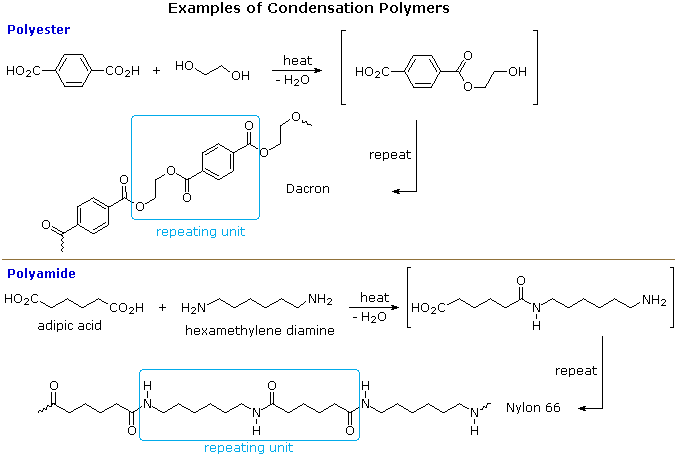
Some Condensation Polymers
| Formula | Type | Components | Tg ºC | Tm ºC |
|---|---|---|---|---|
| ~[CO(CH2)4CO-OCH2CH2O]n~ | polyester | HO2C-(CH2)4-CO2H HO-CH2CH2-OH | < 0 | 50 |
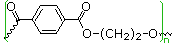 | polyester Dacron Mylar | para HO2C-C6H4-CO2H HO-CH2CH2-OH | 70 | 265 |
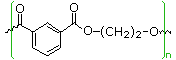 | polyester | meta HO2C-C6H4-CO2H HO-CH2CH2-OH | 50 | 240 |
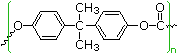 | polycarbonate Lexan | (HO-C6H4-)2C(CH3)2 (Bisphenol A) X2C=O (X = OCH3 or Cl) | 150 | 267 |
~[CO(CH2)4CO-NH(CH2)6NH]n~ | polyamide Nylon 66 | HO2C-(CH2)4-CO2H H2N-(CH2)6-NH2 | 45 | 265 |
~[CO(CH2)5NH]n~ | polyamide Nylon 6 Perlon | 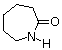 | 53 | 223 |
William ReuschMichigan State University]
4.Molecular Forces
- Elastomers:[Elastomer materials are those materials that are made
of polymers that are joined by chemical bonds(by weakest intermolecular forces), acquiring a final
slightly crosslinked structure.e.g., natural rubber, buna-S, buna-N etc .
The main characteristic of elastomer materials is the high elongation and flexibility or elasticity of these materials, against its breaking or cracking.Depending on the distribution and degree of the chemical bonds of the polymers, elastomeric materials can have properties or characteristics similar to thermosets or thermoplastics, so elastomeric materials can be classified into:-
Thermoset Elastomers - are those elastomer materials which do not melt when heated.
-
Thermoplastic Elastomers - are those elastomers which melt when heated.
- flexible and elastic
Applications:
- Natural rubber - material used in catheters, balloons, medical tubes, elastic thread, and also in some adhesives
- manufacture of elastic clothing
-
- Fibres:[ Fibres belong to a class of polymers which are thread-like and can be woven into fabrics.A few examples of this class are nylon-66, terylene and polyacrylonitrile]properties:
- high tensile strength because the chains possess strong intermolecular forces such as hydrogen bonding
- The fibres are crystalline in nature and have sharp melting points
Applications:
- These are widely used for making clothes, nets, ropes, gauzes, etc
- manufacture of fibre clothing
- Thermoplastics: [A thermoplastic, or thermosoftening plastic, is a plastic
material, a polymer, that becomes pliable or moldable above a specific
temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. (Wikipedia ) . Thus, they can be cast into different shapes by using suitable moulds, e.g.,
- 1 Acrylic
- 2 ABS
- 3 Chlorinated Polyvinyl Chloride
- 4 Nylon
- 5 PLA
- 6 Polybenzimidazole
- 7 Polycarbonate
- 8 Polyether sulfone
- 9 Polyoxymethylene
- 10 Polyetherether ketone
- 11 Polyetherimide
- 12 Polyethylene
- 13 Polyphenylene oxide
- 14 Polyphenylene sulfide
- 15 Polypropylene
- 16 Polystyrene
- 17 Polyvinyl chloride
- 18 Teflon
- Thermosetting plastics: [ A thermosetting polymer (also called a thermosetting plastic or thermosetting resin) is a polymer which becomes irreversibly hardened upon being cured. Curing is caused by the action of heat or suitable radiation and may be promoted by high pressure or the use of a catalyst. It results in extensive cross-linking between polymer chains to give an infusible and insoluble polymer network. A cured thermosetting polymer is called a thermoset.e.g;
- Polyester resin .
- Polyurethanes
- Polyurea/polyurethane
- Vulcanized rubber.
- Bakelite, a phenol-formaldehyde resin used in electrical insulators and plasticware.
- Duroplast
- Urea-formaldehyde foam used in plywood, particleboard and medium-density fibreboard.
- Melamine resin used on worktop surfaces.[2]
- Diallyl-phthalate (DAP)
- Epoxy resin
- Epoxy novolac resins
- Benzoxazines
- Polyimides and Bismaleimides
- Cyanate esters
- Mold or mold runners (the black plastic part in integrated circuits or semiconductors).
- Furan resins
- Silicone
- Thiolyte
- Vinyl ester resins used for wet lay-up laminating, molding and fast setting industrial protection and repair materials.
A.Polyolefins[A polyolefin is a type of polymers produced from a simple olefin as a monomer.C=C]
- Polythene
- Polystyrene (Styrone)
- Polyvinylchloride (PVC)
- Polypropylene (PP)
- Teflon
- Polyacrylonitrile
- Nylon-66
- Nylon-6
- Phenol-Formaldehyde Polymer
- Melamine-formaldehyde Resin
- Urea-formaldehyde Resin
- Natural Rubber
- 5. Neoprene
- Buna-N
- Polymethylmethacrylate (PMMA)
- Glyptal
- Terylene (Dacron)
Molecular masses of Polymers
.....................................
Biopolymers
.........................................
Biodegradable Polymers
..........................................
Some Commercially important Polymers
....................................................
.....................................
Biopolymers
.........................................
Biodegradable Polymers
..........................................
Some Commercially important Polymers
....................................................
See also
References
External links
- https://en.wikipedia.org/wiki/Polymer
- http://pediaa.com/difference-between-polymer-and-macromolecule/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Polymers/Addition_Polymers
- https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/polymers.htm
- https://www.adhesiveandglue.com/elastomer.html


No comments:
Post a Comment